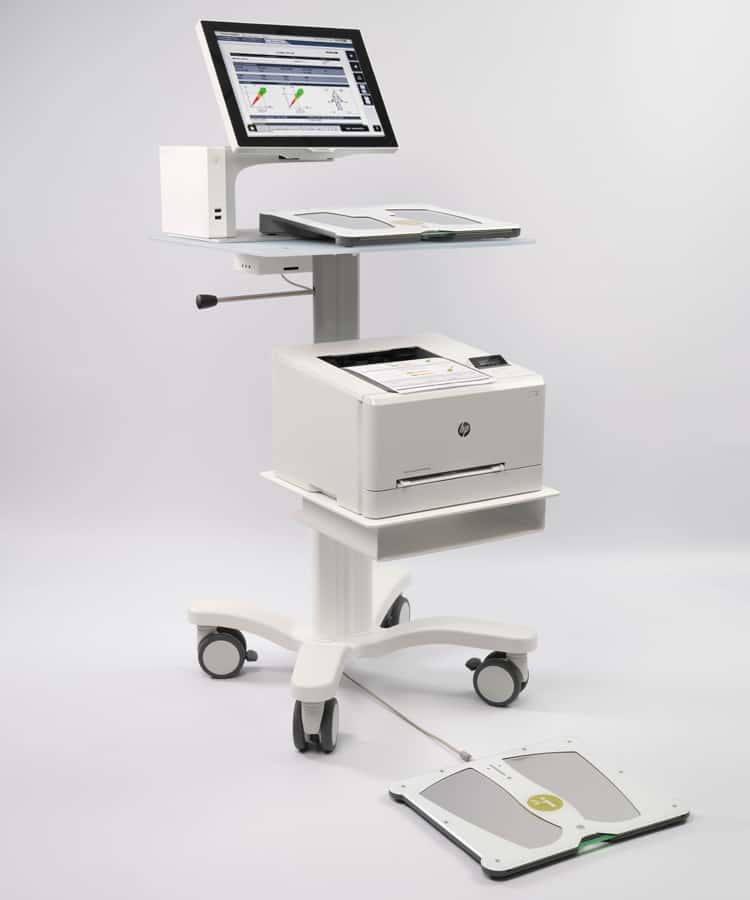
About SUDOSCAN
SUDOSCAN is a medical device that offers a stimulation of the sweat glands that assess small nerve fibers

Diabetes
SUDOSCAN provides a better management of diabetes complications such as neuropathies to prevent diabetic foot.

Neurology
SUDOSCAN has demonstrated its efficacy in painful neuropathies, transthyretin amyloidosis, Sjogren’s Syndrome, Hepatitis C, Fabry disease…
Clinical Evidence for SUDOSCAN
SUDOSCAN Is Becoming a Reference Test in Many Countries.
Commercialized worldwide with more than 5000 devices sold.
CE certified Cleared for use by the US FDA, Registered and distributed in 34 countries Included the European Network for TTR-FAP Amyloidosis, The German guidelines for Diabetes management, The Latin America Diabetes association, And more…
SUDOSCAN Benefits

Fast
No patient preparation
Results in 3 minutes
Easy-to-read critical data points to help physicians reach a diagnosis

Secure
Non-invasive
No fasting
Easy to operate
CE and FDA approvals

Accurate
Reproducible quantitative results
Independent from environmental conditions
Backed by evidence-based research
150 peer-reviewed journals publications